Benzoic Acid Manufacturing Process 14523u
This document was ed by and they confirmed that they have the permission to share it. If you are author or own the copyright of this book, please report to us by using this report form. Report l4457
Overview 6h3y3j
& View Benzoic Acid Manufacturing Process as PDF for free.
More details h6z72
- Words: 450
- Pages: 2
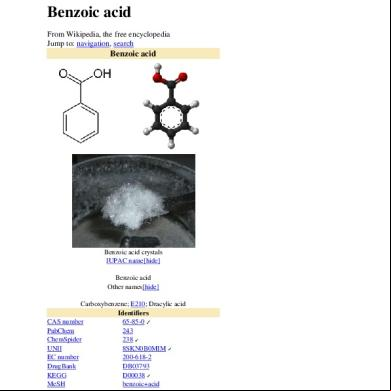
Benzoic acid manufacturing process According to Maki and Takeda , benzoic acid is produced, worldwide, by liquid-phase oxidation of toluene with molecular oxygen. Originally the oxidation reaction was carried out at 140 °C and ca. 0.2 MPa with a cobalt naphthenate catalyst (0.1%). Other oil-soluble cobalt salts were also used as catalysts. The purity of toluene is critical because sulphur compounds, nitrogen compounds, phenols and olefins inhibit the oxidation reaction. The oxidation reaction is a free-radical chain process. Peroxides are reaction intermediates. In a typical modern process, the oxidation is conducted at 165 °C and 0.9 MPa. The pressure of the liquid discharged from the reactor is reduced to atmospheric, and unreacted toluene is recovered. Benzoic acid is purified by rectification. The bottom residue is extracted to recover the cobalt catalyst. In the oxidation reaction, several by-products are formed: benzaldehyde, benzyl alcohol and benzyl benzoate. Other esters, including benzyl formate and benzyl acetate, are also present. Biphenyl and methyl biphenyls are formed in smaller amounts. Small amounts of phthalic acid can also be present. For food and pharmaceutical uses, benzoic acid is upgraded by further processing. Sublimation, recrystallisation and neutralization processes have been proposed. To remove phthalic acid, whose presence is not allowed for food uses, treatment with amines and rinsing is required. According to industry, ‘production of benzoic acid is by liquid-phase oxidation of toluene in the presence of a cobalt catalyst. Air is the source of oxygen in this free radical reaction. Reaction by-products include benzaldehyde, acetic acid, formic acid and benzyl alcohol. Crude benzoic acid is recovered by distillation’ and ‘for sodium benzoate production sodium hydroxide (caustic soda) is added to benzoic acid’. 13)
Sodium benzoate is produced by the neutralization of benzoic acid with sodium hydroxide . Potassium benzoate is obtained in high yield by reacting an aromatic hydrocarbon solution of benzoic acid, such as that which is obtained from the toluene oxidation process, with potassium hydroxide preferably in concentrated aqueous solution, thereby precipitating solid potassium benzoate . No information was available regarding the manufacture of calcium benzoate; the European Food Safety Authority assumed that it is manufactured similarly, by reaction of benzoic acid with calcium hydroxide, or other calcium salt. The European 14)
15)
Food Safety Authority noted that if limestone is the source of calcium carbonate which is used in the production of calcium benzoate, then calcium benzoate could be contaminated with aluminium. In its opinion on the re-evaluation of calcium carbonate (E 170) as a food additive , the European Food Safety Authority noted that limestone may contain aluminium at concentrations up to 190 mg/kg. Therefore, specifications for the maximum level of aluminium in calcium benzoate may be required. 16)
Sodium benzoate is produced by the neutralization of benzoic acid with sodium hydroxide . Potassium benzoate is obtained in high yield by reacting an aromatic hydrocarbon solution of benzoic acid, such as that which is obtained from the toluene oxidation process, with potassium hydroxide preferably in concentrated aqueous solution, thereby precipitating solid potassium benzoate . No information was available regarding the manufacture of calcium benzoate; the European Food Safety Authority assumed that it is manufactured similarly, by reaction of benzoic acid with calcium hydroxide, or other calcium salt. The European 14)
15)
Food Safety Authority noted that if limestone is the source of calcium carbonate which is used in the production of calcium benzoate, then calcium benzoate could be contaminated with aluminium. In its opinion on the re-evaluation of calcium carbonate (E 170) as a food additive , the European Food Safety Authority noted that limestone may contain aluminium at concentrations up to 190 mg/kg. Therefore, specifications for the maximum level of aluminium in calcium benzoate may be required. 16)